Introduction to High Throughput Drug Screening
What is High Throughput Drug Screening (HTS)?
High throughput drug screening (HTS) is a modern method employed in pharmaceutical and biotechnology research to rapidly test large numbers of compounds for their potential to interact with a biological target. This powerful technique allows researchers to assess the biological activity of thousands of different compounds in a short amount of time, significantly accelerating the drug discovery process.
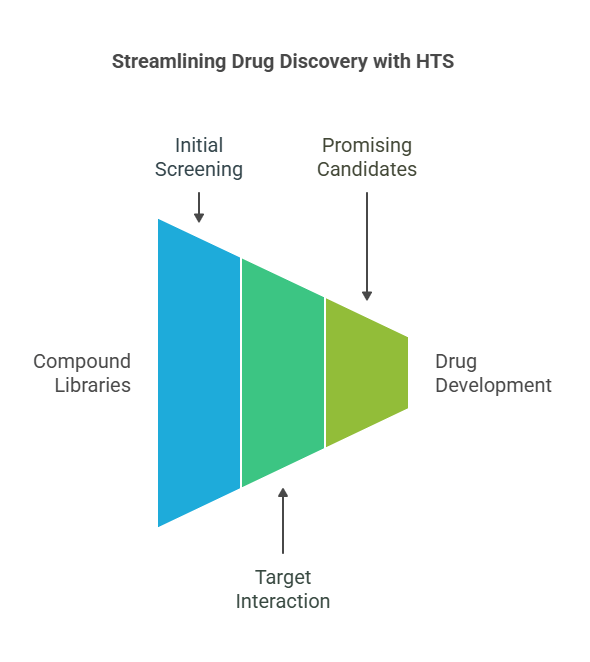
HTS is typically used in the early stages of drug development to identify promising drug candidates that may have therapeutic potential. The goal is to quickly evaluate vast compound libraries to identify molecules that exhibit the desired biological activity, whether it be inhibition of an enzyme, binding to a receptor, or influencing other cellular processes.
The Importance of HTS in Modern Drug Discovery
The drug discovery process is often long, expensive, and resource-intensive. Traditionally, screening compounds for their biological activity was a time-consuming task that required extensive manual labor. However, the advent of HTS has changed the landscape of drug development by enabling pharmaceutical companies and biotech firms to rapidly test a vast array of compounds in parallel, thereby reducing time and costs.
HTS plays a vital role in drug discovery by providing early identification of hits—compounds that show promise in interacting with a disease target. This process allows researchers to focus their efforts on the most promising compounds, helping to identify lead candidates that can move forward to preclinical testing and, eventually, clinical trials.
How Does High Throughput Drug Screening Work?
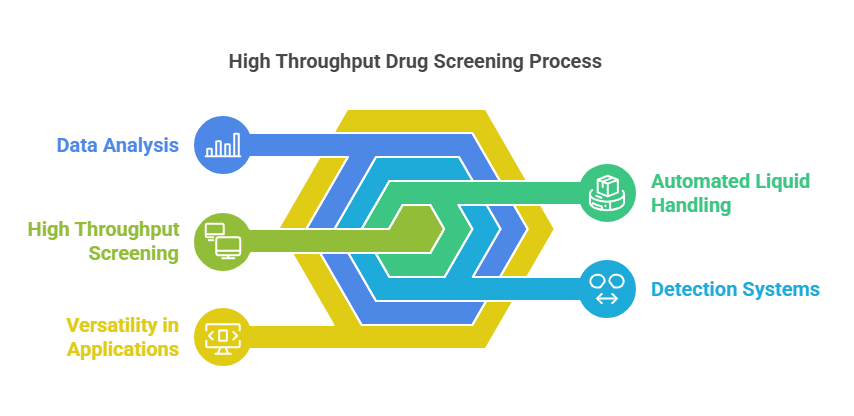
High throughput drug screening utilizes advanced automated systems, robotics, and specialized technologies to test large numbers of compounds in a streamlined manner. The process is built around the use of microplates, which contain multiple wells, each designed to hold small volumes of compounds, biological samples, or assay reagents. These plates can have up to 1,536 wells, allowing researchers to test a large number of compounds in parallel.
The key steps in HTS include:
- Automated Liquid Handling: Robotic systems dispense the compounds into the wells of a microplate, minimizing human error and increasing throughput.
- Detection Systems: After the compounds are added, detection systems such as fluorescence or luminescence readers measure the biological response of each sample in the wells.
- Data Analysis: The results from thousands of tests are collected and analyzed using sophisticated software tools, helping researchers identify promising compounds that exhibit the desired biological activity.
HTS can be applied to a variety of biological targets, including enzymes, receptors, proteins, and genetic material. The versatility of HTS makes it a powerful tool in drug discovery, helping researchers to tackle complex diseases with high unmet medical needs.
Common Types of Assays in HTS
HTS uses a range of assay types to evaluate the biological effects of compounds, depending on the nature of the target being studied. Common assays include:
- Cell-based Assays: These assays use living cells to evaluate a compound’s effect on cell function, growth, or survival. They are ideal for studying diseases where cellular responses are key, such as cancer or neurological disorders.
- Biochemical Assays: In these assays, purified proteins or enzymes are used to measure the effect of compounds on specific biochemical reactions. They are commonly used for targets like enzymes or kinases.
- Genetic Assays: These assays examine how compounds interact with genetic material, such as DNA or RNA. They can be used to investigate gene expression or gene regulation, making them valuable in cancer and genetic disease research.
High Throughput Screening vs. Traditional Drug Screening Methods
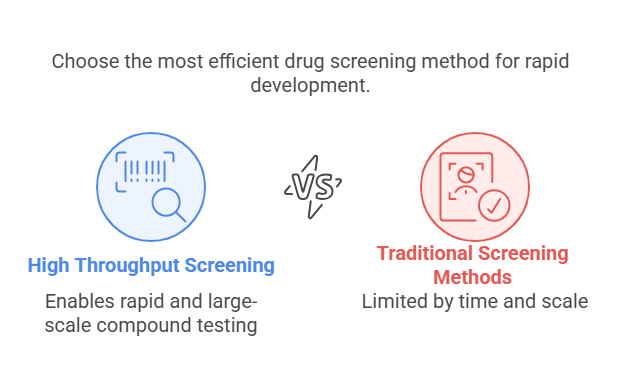
Traditional drug screening methods typically involve testing compounds one at a time, which can be a lengthy and labor-intensive process. These methods are also limited by the number of compounds that can be tested within a given timeframe.
In contrast, HTS leverages automation to screen vast libraries of compounds in parallel, significantly increasing the number of compounds that can be tested within a short period. By using robotic systems, liquid handling automation, and microplates, HTS can test thousands or even millions of compounds, making it a much faster and more efficient process than traditional methods.
HTS provides several distinct advantages over traditional screening, such as:
- Speed: HTS allows researchers to evaluate large compound libraries in days or weeks rather than months or years.
- Scale: The ability to test thousands to millions of compounds at once allows for a broader scope of research.
- Precision: Automation ensures that the screening process is precise, reducing human error and increasing reproducibility.
By using HTS, pharmaceutical and biotech companies can quickly identify potential drug candidates and move them forward in the drug development process more efficiently than ever before.
The Process of High Throughput Drug Screening
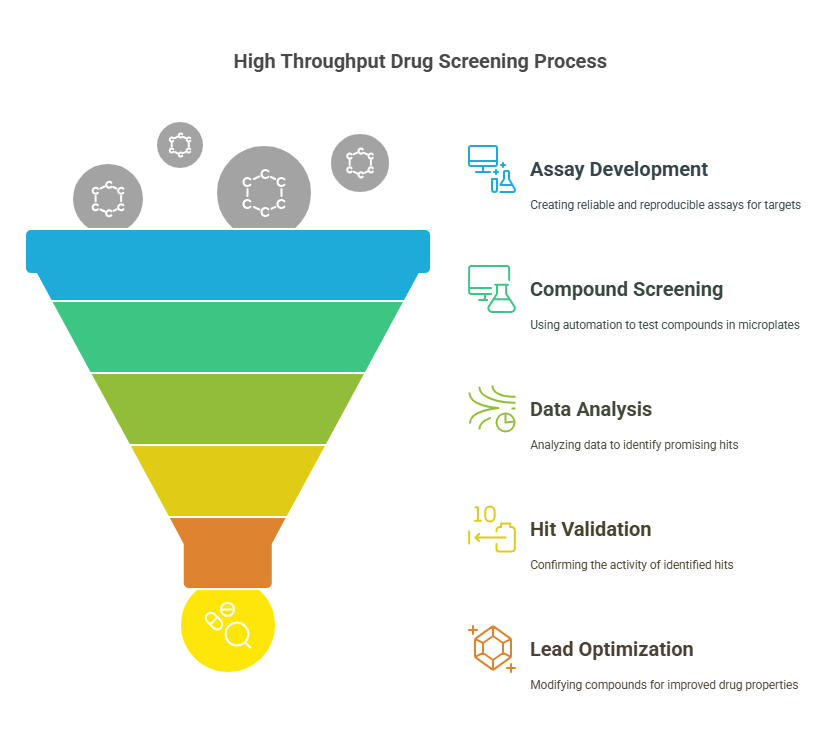
Step-by-Step Process of High Throughput Drug Screening
The process of high throughput drug screening (HTS) involves several key stages, each critical to ensuring the successful identification of promising drug candidates. These stages are designed to efficiently handle a large volume of compounds while maintaining the accuracy and reliability of results. Below is an overview of the HTS process:
- Compound Library Preparation
- The first step in HTS is the preparation of a compound library, which contains thousands or even millions of compounds that will be screened for biological activity. These compounds can be derived from a variety of sources, including synthetic compounds, natural products, and previously identified bioactive molecules.
- The compound library is carefully cataloged and organized to ensure that each compound can be easily accessed and tested during the screening process. Libraries may include a wide range of chemical structures to ensure diversity in the potential drug candidates.
- Assay Development
- In this stage, researchers develop assays that are tailored to the biological target of interest. The choice of assay depends on the type of target, such as a protein, receptor, or enzyme.
- Assay development involves determining the optimal conditions for testing compounds, including reagent selection, assay buffers, incubation times, and detection methods (e.g., fluorescence, luminescence, or absorbance).
- It is crucial that assays are highly reproducible and capable of producing reliable, quantitative data to identify hits (compounds that show promising biological activity).
- Screening the Compound Library
- Once the compound library and assays are ready, the screening process begins. Automated systems, including robotic liquid handlers, are used to add compounds from the library into microplates, typically with 96, 384, or 1,536 wells.
- Each well contains a small volume of compound solution, biological samples, or reagents. The plates are then incubated to allow the compounds to interact with the target in the assay.
- Detection systems such as fluorescence or luminescence readers are used to measure the biological response in each well, providing real-time data on the activity of each compound.
- Data Collection and Analysis
- The results from each assay are collected, generating massive amounts of data. Modern HTS systems use specialized software to record and analyze the data, providing information on the biological activity of each compound.
- Data analysis helps identify “hits”—compounds that exhibit a desired effect, such as inhibition of an enzyme or binding to a receptor.
- Sophisticated data analysis tools and bioinformatics methods are used to handle the high volume of data generated by HTS. This can involve statistical analysis, pattern recognition, and machine learning to refine the results and identify the most promising compounds.
- Hit Identification and Validation
- Once hits are identified, they are typically validated through secondary assays to confirm their activity and ensure the results are reliable. These follow-up assays are usually designed to be more specific and precise.
- If a compound passes validation, it can move on to the next stages of drug development, including lead optimization, preclinical testing, and clinical trials.
- Lead Optimization and Further Testing
- After identifying hits, researchers work on optimizing them into “lead compounds” that are more potent, selective, and suitable for further development.
- In this phase, the chemical structure of the lead compounds may be modified to improve their efficacy, reduce toxicity, and enhance their drug-like properties.
High Throughput Screening vs. Other Drug Screening Methods
HTS is considered a highly efficient and scalable approach compared to traditional drug screening methods. Below is a comparison between HTS and other screening methods:
| Screening Method | High Throughput Screening (HTS) | Low Throughput Screening | Virtual Screening |
|---|---|---|---|
| Speed | Very fast, screens thousands to millions of compounds in days | Slow, screens compounds one at a time | Fast, computational analysis based on molecular docking |
| Scale | Large-scale, handles thousands or millions of compounds | Small-scale, typically handles fewer compounds | Large-scale, handles large compound libraries |
| Automation | Highly automated with robotics, liquid handling, and detection | Manual or semi-automated | Fully automated via computer models |
| Data Output | High volume, quantitative, and reproducible data | Limited data from individual experiments | Predictive data based on computational models |
| Target Complexity | Can screen for complex biological targets | Suitable for simpler assays | Useful for predicting interactions but not always precise |
As shown, HTS offers the advantage of speed, scale, and automation, making it the most efficient approach when large compound libraries need to be screened. Other methods, like low throughput screening, are slower and more limited in their capacity to process data.
Role of Automation and Robotics in HTS
Automation and robotics are fundamental to the success of high throughput drug screening. By employing robotic liquid handlers, automated compound dispensers, and detection systems, HTS eliminates the need for human intervention in most aspects of the process. This significantly increases throughput, reduces errors, and ensures reproducibility.
Robotics allow researchers to process a vast number of compounds in a fraction of the time it would take using manual methods. This efficiency is critical in the fast-paced world of drug discovery, where time-to-market can be a competitive advantage.
In addition, automation provides researchers with greater flexibility to scale the screening process. Whether testing a few thousand compounds or millions, the automated systems can adapt to the needs of the project, handling larger volumes without compromising accuracy.
Rapid Hire Solutions and HTS Technology
Rapid Hire Solutions is a trusted partner for companies seeking to implement or enhance their HTS capabilities. As an industry leader in employment and background screening, Rapid Hire Solutions also works with biotech and pharmaceutical organizations to streamline their research and drug discovery processes. By offering a range of solutions to support the automation of data collection and screening, Rapid Hire Solutions helps businesses increase efficiency, reduce operational costs, and ensure compliance with industry standards.
Their expertise in implementing cutting-edge technologies, including robotics and automation, allows organizations to focus on advancing their research while Rapid Hire Solutions handles the complex data management and logistical components of HTS.
Legal Aspects of High Throughput Drug Screening
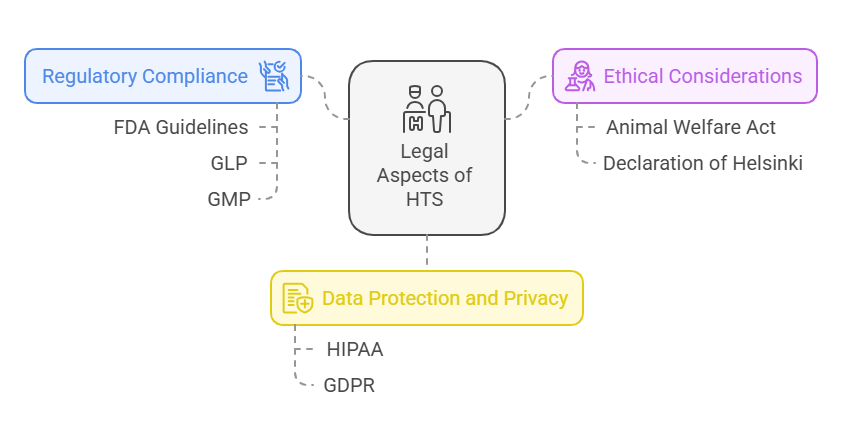
High throughput drug screening (HTS) is governed by various regulatory and ethical frameworks to ensure the safety, efficacy, and legality of drug discovery. The legal aspects surrounding HTS primarily focus on patient safety, data protection, and the ethical use of technology in pharmaceutical research. Below are key legal considerations:
1. Regulatory Compliance
HTS falls under several regulatory guidelines that govern drug development, particularly in the United States. The Food and Drug Administration (FDA) is responsible for ensuring that drug candidates undergo rigorous testing and meet safety standards before entering the market. HTS is an essential part of this process, as it enables researchers to identify compounds that may have therapeutic potential.
- Good Laboratory Practices (GLP): GLP guidelines are critical in ensuring that HTS processes are conducted reliably and consistently. These guidelines ensure that all equipment, procedures, and personnel involved in HTS adhere to standard operating procedures that support the production of credible data.
- Good Manufacturing Practices (GMP): If a lead compound moves into clinical trials or manufacturing, GMP ensures that it is produced in a safe and effective manner. HTS technologies often work in tandem with GMP to ensure that early-stage research transitions into the clinical phase smoothly.
2. Ethical Considerations in Drug Testing
As with all research in drug discovery, ethical considerations are of utmost importance in HTS. One of the primary concerns is ensuring that animal and human testing follow the Animal Welfare Act and the Declaration of Helsinki, which govern ethical treatment and rights in research involving animals and humans. Researchers must also ensure that their HTS practices do not exploit vulnerable populations or lead to the misuse of data for non-research purposes.
3. Data Protection and Privacy
The Health Insurance Portability and Accountability Act (HIPAA), along with the General Data Protection Regulation (GDPR) in the EU, provides legal protection for personal health information. This is especially important in clinical trials or when screening compounds that will eventually be tested on humans. All data collected through HTS, particularly data involving human samples or genetic information, must be securely stored and protected from unauthorized access.
Additionally, bioinformatics and computational data generated during HTS are subject to data security measures to prevent cyber threats and ensure the confidentiality of proprietary research.
Frequently Asked Questions about High Throughput Drug Screening
What is the main advantage of high throughput drug screening over traditional methods?
The primary advantage of high throughput drug screening (HTS) over traditional methods is its ability to screen thousands to millions of compounds in a very short period. Traditional methods often involve manually testing smaller numbers of compounds, which can be time-consuming and labor-intensive. HTS, on the other hand, uses automation and robotics to streamline the process, drastically reducing the time and labor required to identify potential drug candidates.
How accurate are the results from high throughput screening?
The accuracy of HTS results depends on the quality of the assays, the automation equipment, and the data analysis systems used. HTS has the potential to produce highly accurate results, but it is important to follow best practices in assay development, automation, and data validation. While HTS identifies hits (compounds that show activity), further validation through secondary assays is necessary to confirm the initial results.
Can high throughput drug screening be used for all types of diseases?
Can high throughput drug screening be used for all types of diseases?
HTS is a versatile tool that can be used for a wide range of diseases, including cancer, neurological disorders, infectious diseases, and cardiovascular conditions. The success of HTS depends on the availability of suitable biological targets for the disease, and researchers must tailor the screening assays to the specific type of disease or condition being studied.
What are the key challenges in implementing high throughput screening?
While HTS offers significant advantages in drug discovery, there are challenges associated with its implementation. These challenges include the high initial cost of setting up automated systems, the need for specialized training and expertise, and the requirement for vast compound libraries. Additionally, interpreting the vast amount of data generated by HTS can be complex, requiring robust data analysis tools and bioinformatics support
How long does it take to complete a high throughput drug screening process?
The timeline for completing an HTS process varies depending on the size of the compound library and the complexity of the assays. In general, HTS can screen thousands of compounds within a few days to a couple of weeks. However, the entire drug discovery process, from initial screening to lead optimization, can take several months or even years before reaching clinical trials.
Conclusion
High throughput drug screening has revolutionized the field of pharmaceutical research by significantly accelerating the identification of potential drug candidates. Through automation, robotics, and data analysis, HTS enables researchers to screen vast libraries of compounds efficiently and accurately, bringing promising drugs to market faster.
In addition to its technological advancements, HTS also operates within a strict regulatory framework that ensures safety, efficacy, and ethical standards in drug discovery. Understanding the process, legal considerations, and advantages of HTS is crucial for pharmaceutical and biotech companies aiming to stay competitive in the rapidly evolving industry.
Rapid Hire Solutions is an excellent resource for organizations looking to implement or enhance their HTS capabilities. By offering automation and expertise in screening processes, Rapid Hire Solutions helps streamline workflows, increase efficiency, and ensure compliance with industry regulations.
In conclusion, high throughput drug screening is a transformative technology in modern drug discovery. It helps pharmaceutical companies find promising candidates for diseases and conditions that previously lacked effective treatments. As the technology continues to evolve, its potential for accelerating drug development and improving public health outcomes remains immense.
